Addition of Hydrogen Halides to Alkenes Mechanism
With such capabilities they can. Formation of alkenes.
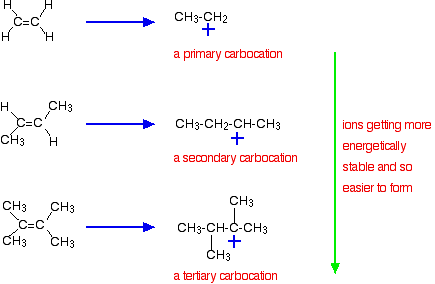
9 2 Addition Of Hydrogen Halides To Symmetrical Alkenes Chemistry Libretexts
The primary alcohols elimination reactions follow the E2 mechanism whereas the secondary and tertiary alcohols elimination reaction follows the E1 mechanism.
. CHCl COEt ACN CoEt AcOEt CN -78C 8h 25 75 When cyclopentadiene is added in excess the reaction is pseudo-1 order with respect to the fumaric nitrile ester. Reaction Mechanism Click Here for Sample Questions The haloalkanes or aryl halides with sp 3 or sp 2 hybridised carbon atoms when reacted with Magnesium metal give Grignard reagent which is an organometallic compound. The reduction product is a minor.
The S N 2 doesnt happen for secondary alcohols. Gabriel Phthalimide Synthesis was discovered by a German chemist named Siegmund Gabriel. In case of unsymmetrical alkenes the addition reaction takes place in accordance with Markovnikovs rule Unit 13 Class XI.
For example if the Rgroups on the beta-carbon enhance the acidity of that hydrogen then substantial breaking of CH may occur before the other bonds begin to be affected. For instance alkanes alkynes or alkenes the amount of bonded hydrogen decreases in alkenes and alkynes. Substantial amounts of the expected arylation product were indeed obtained by irradiating aryl halides in the presence of N-methylpyrrole and catalytic amounts of PDI.
However often the two are used interchangeably because the. You will find it - Its all here. Only one textbook in this admittedly incomplete sample mentions the S N i mechanism at all.
Drawing formulas from names. Thus the carbonyl carbon and the three atoms attached to it lie in the same plane and the π-electron cloud is above and below this plane. In addition to primary amines secondary amines led to reductive coupling with nitriles and provided tertiary chiral amines in up to 85 yields and 99 ee Fig.
The steps that are involved. Mechanism The mechanism of the reaction involves the following three steps. Represented by R-Mg-X where R is an alkyl or aryl group while X is a halogen the Grignard reagent easily forms a carbon-carbon.
Alkenes having four or more carbon atoms can form diverse structural isomersMost alkenes are also isomers of cycloalkanesAcyclic alkene structural isomers with only one double bond follow. In addition it is worth noting that formation of hydrogen halides during the reaction can deactivate the catalyst and that hydrodehalogenation is delayed or even stopped at elevated concentrations of hydrogen halide. Matching alcohols to their names I.
Drawing formulas from names. Mechanism of Dehydration of Alcohols. In addition the oxygen atom also has two non bonding electron pairs.
In four textbooks where SOCl 2 is mentioned the reaction is shown as proceeding through an S N 2 mechanism. Theres no warning sign saying wait. Products 214 to 215.
Drawing alkene formulas from names. The Gabriel synthesis is a chemical reaction used to obtain primary amines from primary alkyl halides. C 5 H 10.
C 2 H 4. If its not in the textbook chances are it. The ability of hydrocarbons to bond to themselves is known as catenation.
Organic Chemistry Study Materials Practice Problems Summary Sheet Guides Multiple-Choice Quizzes. Alkenes react with water in the presence of acid as catalyst to form alcohols. We therefore selected as the reaction partner N-heterocyclic pyrroles which were found to have high reaction rates in the addition of radicals.
1212 Structure of the Carbonyl. Drawing alcohol formulas. From alkenes i By acid catalysed hydration.
The bond angles are approximately 120 as expected of a trigonal coplanar structure Figure 121. C 4 H 8. The following Diels-Alder cycloaddition was recently performed by Mukherjee et al.
This is mainly due to the self-bonding or catenation of carbon that prevents the complete saturation of the hydrocarbon by the formation of double or triple bonds. 1-butene 2-butene and isobutylene. The extent of deactivation was generally determined to depend on the particular hydrogen halide formed following the sequence of BrH ClH.
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. Drawing alkyne formulas from names. Similarly groups that favor ionization of the halogen may generate a transition state with substantial positive charge on the alpha-carbon and only a small degree of CH breaking.
C 3 H 6. Notably for products 210 and 214 12 to 14 racemization is observed which can be explained by the comparably high acidity of the α-hydrogen atom in the case of the starting 2. Dehydration of alcohols follows the E1 or E2 mechanism.
Drawing formulas from names. It is a subset of reactions that very closely resembles the insertion reactions and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. Visit Friedel Crafts Reaction for an in-depth explanation of the reaction details and mechanism.
Basically it follows a 3-step mechanism.
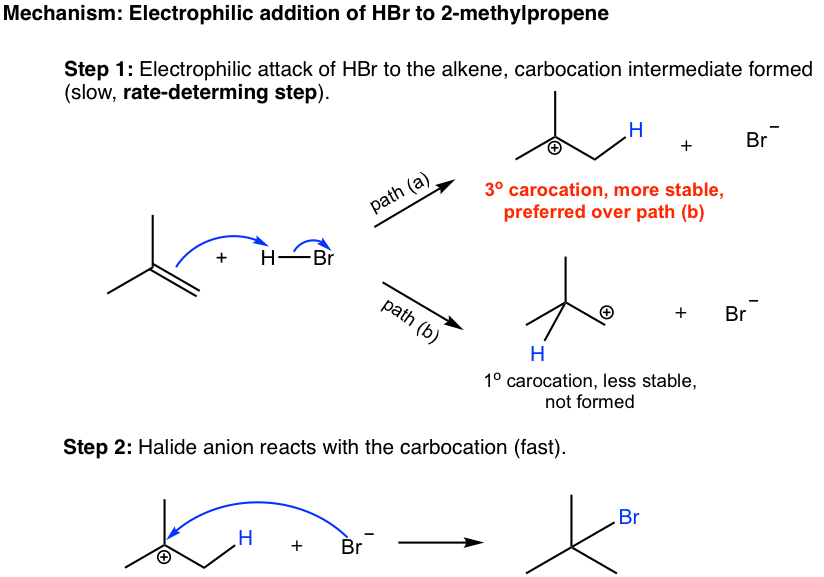
10 2 Reactions Of Alkenes Addition Of Hydrogen Halide To Alkenes Organic Chemistry I
Addition Of Hydrogen Halides To Alkenes Chemgapedia
Addition Of Hydrogen Halides To Alkenes Chemgapedia

Electrophilic Addition Of Hydrogen Halides To Alkenes Youtube
Comments
Post a Comment